Our world is facing an unprecedented crisis of antimicrobial resistance (AMR) in which bacteria and other microbes are developing resistance to medications like antibiotics that were once effective against them. As a result of AMR, even common infections are developing the potential to become deadly, leading the WHO to declare AMR a “silent pandemic,” and consider it one of the top ten global public health threats to humanity in the 21st century.
A number of promising strategies are now being employed to counter the threat of AMR, and among them phage therapy is noteworthy1. This promising, re-emerging approach utilizes bacteriophages—viruses that exclusively infect and kill bacteria. This beneficial use of viruses is similar to that of oncolytic viruses, which are used to target and destroy cancer cells.
Phage therapy isn’t new; its history dates back to the early 20th century with the independent discovery of bacteriophages by Frederick Twort and Félix d’Hérelle2. Before antibiotics started to become widely available in the West, phage therapy was commonly used in Eastern Europe, notably at the Eliava Institute in Georgia. Before the emergence of the AMR crisis, Western medicine largely abandoned phages, but now phage therapy is gaining a renewed and fervent interest as a highly specific, effective, and environmentally friendly approach to combat antibiotic-resistant bacteria3.
Understanding bacteriophages
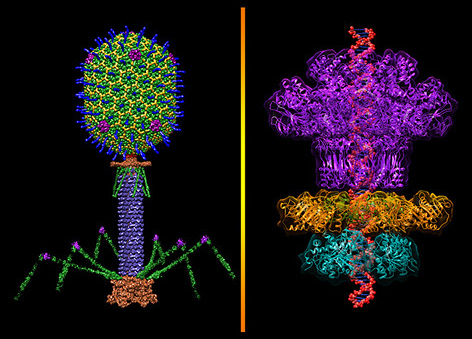
Bacteriophages, often simply called phages, are viruses that exclusively infect bacteria and are found wherever bacteria thrive—in soil, water, sewage, and even within the human body4. The fundamental structure of phages consists of genetic material (either DNA or RNA) encased within a protective protein shell called a capsid.
Phage life cycles
Phages primarily operate through two distinct life cycles: the lytic cycle and the lysogenic cycle5.
Lytic cycle (virulent phages)
This cycle describes how virulent phages kill their bacterial hosts. First, the phage binds specifically to unique receptor sites on the surface of a target bacterium, and then injects its genetic material into the bacterial cell, leaving its capsid outside. The genetic material of the phase then hijacks the cellular machinery of the bacterium and redirects it to synthesize phage components like proteins and nucleic acids. After the synthesis, the phage components self-assemble to form complete progeny phage particles. Following this, the phage produces enzymes, such as lysins and holins, that trigger the lysis of the bacterium by degrading the bacterial cell wall from within. Consequently, hundreds of new, infectious phage particles get released, ready to infect other bacteria.
Lysogenic cycle (temperate phages)
In this cycle, the temperate phage integrates its DNA into the bacterial chromosome, forming a prophage that then replicates along with the bacterial DNA during normal cell division, lying dormant. Under certain stress conditions, like UV radiation, the prophage can excise itself from the bacterial chromosome and enter the lytic cycle.
Virulent phages that undergo the lytic cycle are crucial for therapeutic applications as they kill the target bacteria, a primary goal of phage therapy. Temperate phages are generally avoided as they do not immediately kill bacteria, and because they sometimes carry and transfer virulence factors or antibiotic resistance genes, worsening an infection6.
Phage specificity
Phages typically recognize and infect only very specific bacterial strains, or sometimes a narrow range of strains within a species7. This gives phage therapy a significant advantage over broad-spectrum antibiotics, as it can eliminate pathogenic bacteria without disrupting the beneficial bacteria that make up the human microbiome. On the flip side, such specificity requires precise identification of the infecting bacterial strain, necessitating rapid diagnostic capabilities8. It also requires the availability of potentially large, well-characterized phage libraries to choose a specific phage (or mixture of phages) that can effectively lyse the strain, which is crucial for the success of phage therapy9.
Mechanisms of action in phage therapy
Targeted bacterial lysis
The primary and most direct mechanism is the highly targeted bacterial lysis that occurs during the lytic cycle. In addition, phages self-amplify in situ as long as their target bacteria are present, thereby enabling sustained therapeutic levels, unlike antibiotics that are consumed or metabolized10.
Biofilm penetration and disruption
Biofilms, which encase the bacterial community in a protective extracellular matrix, are notoriously resistant to both antibiotics and host immune defenses. Many phages can penetrate such matrices, either through passive diffusion, active motility, or by producing enzymatic depolymerases that degrade the matrix components11. Once inside, phages can infect and lyse bacteria within the biofilm. In cases where phages disrupt the biofilm structure, the remaining bacteria also get exposed to host immune cells as well as co-administered antibiotics12,13.
Immune system modulation
Phages don’t directly target host cells, but their interaction with bacteria can indirectly influence the host’s immune system14.
- The lysis of bacterial cells by phages often releases certain bacterial components that can trigger the host’s innate immune response, thereby contributing to overall bacterial clearance. For example, lysis of Gram-negative bacteria releases pathogen-associated molecular patterns like lipopolysaccharide (LPS) that can trigger such an immune response.
- Phages, being foreign entities, can be recognized by the host immune system, leading to inflammatory responses, which have the potential to prime or enhance the host’s natural defenses against the pathogenic bacteria.
Key advantages of phage therapy
- The distinct mechanisms of action of phages make them highly effective against multi-drug resistant (MDR) and extensively drug-resistant (XDR) bacterial strains. Phages can also rapidly evolve with their bacterial hosts, which offers a dynamic defense mechanism that adapts to bacterial resistance7.
- High selectivity allows phages to target pathogenic bacteria, leaving the patient’s beneficial microbiome largely undisturbed.
- The fact that phages continue to replicate as long as the target bacteria are present, helps sustain therapeutic levels at the infection site10.
- The ability of phages to effectively penetrate and sometimes disrupt biofilms is crucial for chronic infections.
- Phages can be administered via a wide array of routes, including oral, topical, intravenous, aerosol inhalation, or direct instillation into infected cavities15.
- New phages can be readily isolated from diverse natural sources like sewage, soil, and wastewater for therapeutic development.
Disadvantages and challenges of phage therapy
Effective phage therapy requires the maintenance of extensive collections of well-characterized phages and the development of rapid diagnostic capabilities to quickly identify the causative pathogenic bacteria8,9.
Bacteria can also develop resistance to phages by altering the surface receptors that phages use for attachment or activating their CRISPR-Cas systems that target phage DNA16.
In cases where infections are caused by multiple bacterial species or when the specific strain is not identified, phage cocktails are used. These are mixtures of several different phages, which helps to provide broader coverage and counter the development of bacterial resistance to any single phage17.
Comprehensive data on the pharmacokinetics and pharmacodynamics of phages within the human host remains limited, making it difficult to determine precise dose and optimal administrative schedule18.
As already mentioned, phages can trigger an immune response that can neutralize them; consequently, repeated exposure limits the efficacy of phages. In addition, they can rarely induce allergic reactions. A more significant concern is the issue of endotoxin release during cell lysis. This is relevant in the case of Gram-negative bacterial infections, where the release of endotoxins like LPS can lead to a systemic inflammatory response akin to a Jarisch-Herxheimer reaction19.
The fact that phages are ‘living drugs’ poses unique challenges for quality control, purification, and standardization compared to chemical drugs20. Furthermore, there is a lack of clear and harmonized regulatory frameworks globally21. In addition, a general lack of public awareness and misconceptions about viruses (often only associated with disease) is a clear barrier to acceptance.
Clinical applications and current status
Phage therapy is increasingly being explored and implemented across various fields both in human medicine and beyond.
Human clinical applications
In many Western countries, phage therapy is primarily employed under ‘compassionate use’ protocols for critically ill patients with untreatable MDR and XDR infections22. Such uses have shown promising results across a diverse range of infections:
- Skin and soft tissue infections which include infected burns, diabetic foot ulcers, and chronic wounds where bacterial biofilms are common23.
- Respiratory tract infections, particularly in patients with cystic fibrosis suffering from chronic Pseudomonas aeruginosa infections24.
- Urinary tract infections, especially the recurrent and complicated types caused by resistant strains25.
- Gastrointestinal infections for treating conditions like Clostridioides difficile and other diarrheal diseases26.
- Device-associated infections related to catheters, prosthetic joints, and other medical implants, where biofilms are a major hurdle for antibiotics27.
In addition, an increasing number of clinical trials are being conducted globally to evaluate the safety and efficacy of phage therapy to treat specific infections, with focus on assessing the performance of phage cocktails and combination therapies alongside antibiotics.
Applications beyond human medicine
Beyond human therapeutic use, phages are offering solutions in various sectors:
- Veterinary medicine and aquaculture: For treating bacterial infections in livestock and companion animals, as well as in fish and shellfish farming, to prevent the development and spread of antibiotic resistance to human28,29.
- Agriculture: Phages are being employed as biocontrol agents against bacterial plant diseases from pathogens like Xanthomonas and Pseudomonas30.
- Food Safety: Phage sprays or washes are increasingly used to prevent foodborne illness from pathogens like Salmonella, Listeria monocytogenes, and E. coli O157:H731.
- Environmental bioremediation: Phages can be harnessed to clean up specific bacterial contaminants in contaminated soil or water sources32.
Future directions and perspectives
A critical step forward includes international collaboration for clinical trials, data sharing, and regulatory harmonization for accelerated product development and approval. This includes the establishment of robust “phage banks” with well-characterized and quality-controlled phage preparations and the implementation of good manufacturing practice (GMP) standards for their production.
Advancements in genetic engineering and synthetic biology are aiding researchers to prepare phages that infect a wider range of bacterial strains or even species, resulting in more efficient bacterial killing and higher phage yields. They are also being engineered to be less prone to host immune clearance for improved efficacy. Furthermore, engineered phages can now deliver additional therapeutic payloads, such as antimicrobial peptides, immunomodulators, biofilm-dispersing agents. or even CRISPR-Cas components to directly edit or kill bacterial pathogens from within11,33.
In order to enhance stability, targeted delivery, and overcome physiological barriers, researchers are developing novel delivery systems, including encapsulation techniques using nanoparticles or hydrogels, and optimizing routes like aerosolized delivery for respiratory infections34,35.
The development of rapid diagnostics to quickly identify the infecting pathogen and its specific susceptibility profile to available phages will help to create or select phage cocktails tailored precisely to an individual patient’s infection, an essential goal for personalized medicine8,9.
As research progresses and regulatory pathways mature, this rapidly re-emerging and sophisticated therapeutic strategy is poised to transform our approach to fighting drug-resistant pathogens, offering renewed hope in the ongoing battle for global health.