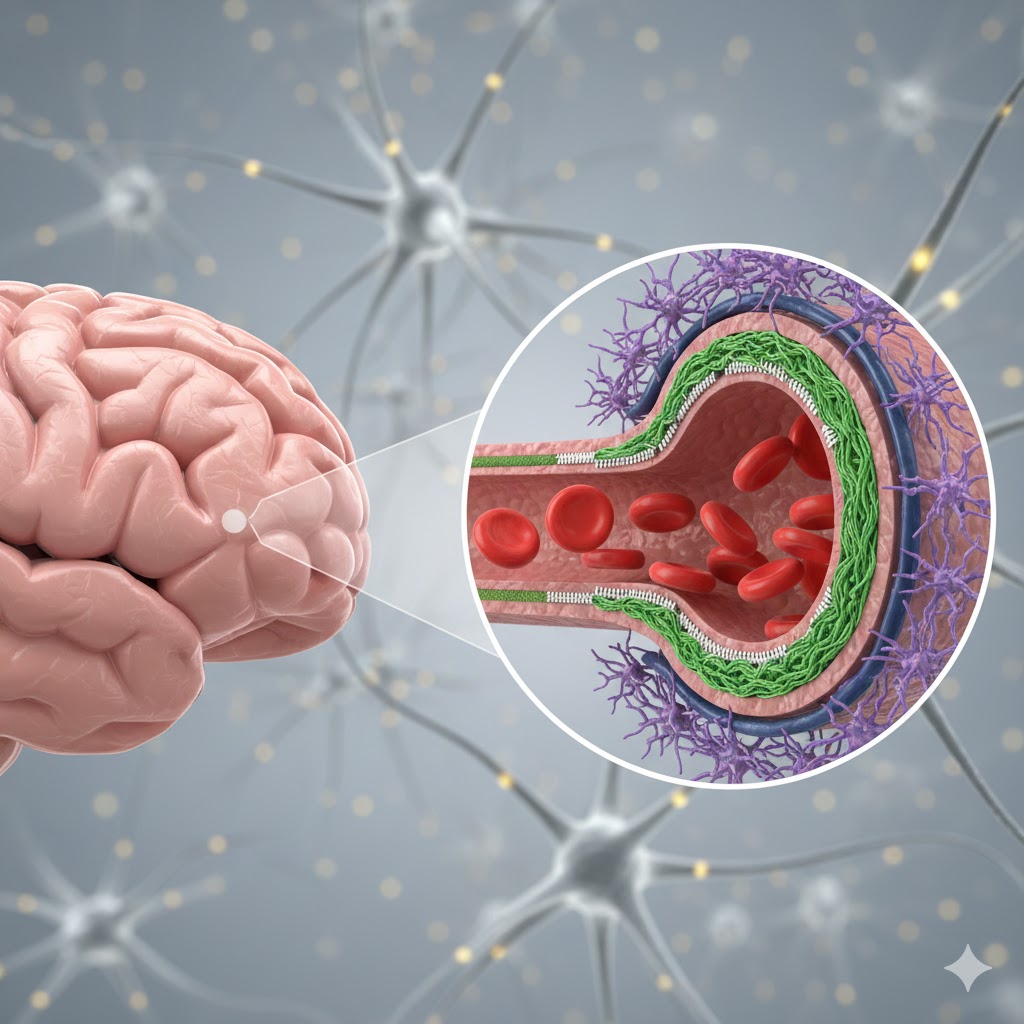
The human brain, the most intricate and vital of our organs, requires an exceptionally stable and protected environment to carry out its functions optimally. This reliability is provided by the Blood-Brain Barrier (BBB), a highly specialized and dynamic neurovascular unit that precisely controls the passage of substances from the bloodstream into the brain1. The BBB is much more than a simple wall; it acts as a sophisticated interface between the central nervous system (CNS) and the peripheral circulation. Its primary purpose is to shield the brain from circulating toxins, pathogens, and harmful fluctuations in blood composition, while simultaneously ensuring the selective entry of essential nutrients. While this remarkable selectivity is critical for maintaining brain homeostasis, it also creates a formidable obstacle to the delivery of therapeutic drugs for many neurological disorders2.
Structure and cellular components of the BBB
The unique function of the BBB arises from the specialized architecture of the brain’s microvessels, where multiple cell types work together.
Endothelial cells are the primary barrier
The insides of brain capillaries are lined by endothelial cells that clearly differ from those seen in other organs1. These cells form very tight junctions—protein complexes known as zonula occludens—that seal the spaces between adjacent cells, and thus restricts the paracellular movement of molecules. Brain capillaries, unlike most peripheral capillaries, lack certain pores called fenestrations and have very few pinocytotic vesicles that limits bulk transport. Their high mitochondrial content reflects the substantial energy required to power selective transport mechanisms.
Pericytes support the barrier
Embedded in the basement membrane and wrapped partially around endothelial cells are certain mural cells, called pericytes1. They are indispensable for the development and function of the BBB because they regulate blood flow, maintain barrier integrity, and stimulate the formation and maintenance of tight junctions.
Astrocytes offer metabolic and structural support
Astrocytes are star-shaped glial cells that project their “end-feet” to almost envelop brain capillaries1. They induce and sustain tight junction formation in endothelial cells, provide metabolic support to neurons and blood vessels, and participate in neurovascular coupling. This neurovascular coupling links neuronal activity to localized blood flow adjustments.
Basement membrane is the structural scaffold
A basement membrane is a specialized extracellular matrix layer composed of proteins such as collagen, laminin, and fibronectin2. It provides structural support and signaling cues that are essential for maintaining the BBB.
Neurons and microglia offer modulation and immunity
Neurons do not form part of the physical barrier, however, they do influence BBB properties through the release of signaling molecules3. The resident immune cells of the CNS, called microglia, also contribute to inflammatory responses and BBB regulation, especially during injury or disease4.
Key functions of the BBB
The complex structure of the BBB is what allows it to perform several crucial protective and regulatory functions.
Physical barrier
Tight junctions prevent harmful molecules and pathogens in the blood from entering the brain’s delicate environment1.
Transport regulation
The BBB selectively permits entry of vital nutrients, like glucose, amino acids, and vitamins, via dedicated transporters while actively exporting metabolic waste and many drugs5.
Enzymatic barrier
Endothelial cells enzymatically degrade or modify potentially harmful substances before they can enter the brain tissue6.
Immune isolation
The BBB restricts entry of peripheral immune cells under normal conditions, thus preventing excessive CNS inflammation7.
Homeostasis maintenance
It regulates ion concentrations, pH, and fluid balance in the brain interstitial fluid, which is essential for optimal neuronal excitability and neurotransmission1.
Transport mechanisms across the BBB
The transport of molecules across the BBB occurs chiefly through paracellular transport, transcellular transport, and efflux pumps.
Paracellular transport
Tight junctions drastically limit movement between endothelial cells, blocking most hydrophilic molecules1.
Transcellular transport
Substances must pass through endothelial cells by1:
- Lipid-mediated diffusion of small, lipophilic molecules such as ethanol and caffeine.
- Carrier-mediated transport (CMT) of essential hydrophilic molecules like glucose via GLUT1 and amino acids via LAT18.
- Receptor-mediated transcytosis (RMT) for larger proteins such as transferrin or insulin, which bind endothelial receptors and are transported across in vesicles8.
- Adsorptive-mediated transcytosis (AMT) for positively charged molecules binding to endothelial surfaces, enabling their uptake8.
Efflux pumps
ATP-driven transporters like P-glycoprotein actively pump many substances out, including therapeutic drugs, back into the bloodstream, thus posing a major obstacle to effective CNS drug delivery1.
Factors influencing BBB permeability
BBB permeability fluctuates with physiological and pathological changes.
Physiological influences
Age affects barrier integrity, with higher permeability in neonates and potential alterations in advanced age9, 10. Circadian rhythms may cause minor daily variations11.
Pathological conditions
The BBB’s tight junctions can be compromised, leading to a disruption in its function. This can be caused by a wide range of conditions, including inflammation, infections (like meningitis), ischemic stroke, traumatic brain injury, and brain tumors12, 13. It can also be a consequence of chronic diseases such as epilepsy, hypertension, and neurodegenerative disorders, including Alzheimer’s, Parkinson’s, and Multiple Sclerosis (MS)14, 15.
Environmental toxins
Exposure to heavy metals, pesticides, and air pollutants may damage BBB integrity16-18.
Pharmaceutical manipulation
Certain drugs like mannitol can transiently and non-specifically open the BBB to facilitate drug delivery, though their risks limit clinical use19.
Role of the BBB in brain health and disease
The BBB is vital for maintaining a stable environment required for brain function, protecting neurons from harmful substances, regulating waste clearance, and supporting neurodevelopment. A compromised BBB can exacerbate disease by allowing toxic molecules or immune cells into the CNS20. This fuels neuroinflammation as seen in MS, Alzheimer’s, and Parkinson’s disease.
Blood-brain barrier disruption is a major contributor to secondary injury after stroke and traumatic brain injury and complicates treatment of brain tumors due to uneven permeability21, 22. Recent studies also suggest a role for subtle BBB dysfunction in psychiatric illness and CNS infection23.
Challenges imposed by the BBB on CNS drug delivery
The BBB is the greatest obstacle to effective drug delivery for neurological diseases:
- Its tight junctions, lack of fenestrations, limited vesicular transport, and active efflux pumps exclude more than 98% of small molecules and almost all large molecule therapies, and this includes antibodies or gene therapies1.
- Many promising drugs fail due to insufficient brain penetration, thus limiting treatment options for disorders like Alzheimer’s, Parkinson’s, and brain cancer24.
- Endothelial metabolism may degrade some compounds before crossing6.
Strategies to bypass or modulate the BBB
To overcome this barrier, various innovative approaches are adopted.
Invasive/disruptive techniques
- Osmotic disruption using hypertonic solutions can temporarily open tight junctions but is risky and non-specific25.
- Direct drug delivery into cerebrospinal fluid via intrathecal or intracerebroventricular injections circumvents the BBB26. However, it is invasive and poses distribution challenges.
- Focused ultrasound combined with microbubbles enables transient, localized, and reversible BBB opening with minimal invasiveness27. It is currently a highly promising clinical technique.
Exploiting endogenous transport pathways
Designing drugs as lipophilic prodrugs or chemically modifying them to enhance passive diffusion helps cross this barrier28. More sophisticated tactics include hijacking CMT, AMT or RMT systems8. One such approach, known as the “Trojan horse” strategy, involves using molecules that mimic natural ligands like transferrin or insulin to ferry drugs across the barrier.
Cell-mediated delivery
Utilizing immune cells such as macrophages or stem cells naturally cross the BBB to deliver therapeutic agents directly into brain tissue29.
Nanoparticles mediated delivery
Encapsulating drugs in liposomes or polymeric nanoparticles, often surface-functionalized with BBB-targeting ligands or coatings, facilitates passage and reduces chances of efflux30.
Efflux pump modulation
Inhibitors of key transporters, such as P-glycoprotein can improve the retention of drugs in the brain31. However, using them poses considerable challenges due to systemic toxicity.
Future directions
Emerging research directions aim to deepen understanding and improve therapeutic delivery:
- Advanced in vitro BBB models, including organoids, organ-on-a-chip and stem cell-derived systems, allow more accurate drug screening and mechanistic studies32, 33.
- In vivo imaging modalities enable real-time monitoring of the BBB integrity and drug penetration34.
- The development of precise, localized technologies seeks to modulate the BBB35. The goal is a reversible and targeted opening that minimizes systemic side effects.
- Artificial intelligence and machine learning help to predict BBB permeability and facilitate rational design of novel carriers or peptides that penetrate the BBB efficiently36.
- Growing appreciation of regional BBB heterogeneity supports the development of site-specific therapies37.
- Researchers are focusing on therapies that restore BBB integrity in diseases where the barrier has broken down. Restoring the BBB is key to limiting neuroinflammation and disease progression.
- Gene therapy vectors capable of crossing the BBB promise future treatment of genetic CNS disorders with targeted delivery38.
Conclusion
The Blood-Brain Barrier essentially serves as our brain’s very own shield, which protects its unique microenvironment from harmful substances while regulating the entry of vital nutrients. Yet, this same barrier poses the most formidable hurdle in treating neurological disorders by blocking the vast majority of drugs from reaching their targets within the CNS. Overcoming this challenge through deeper knowledge of BBB biology and innovative drug delivery is central to unlocking effective therapies for a broad spectrum of devastating brain diseases, from Alzheimer’s and Parkinson’s to stroke and brain tumors.