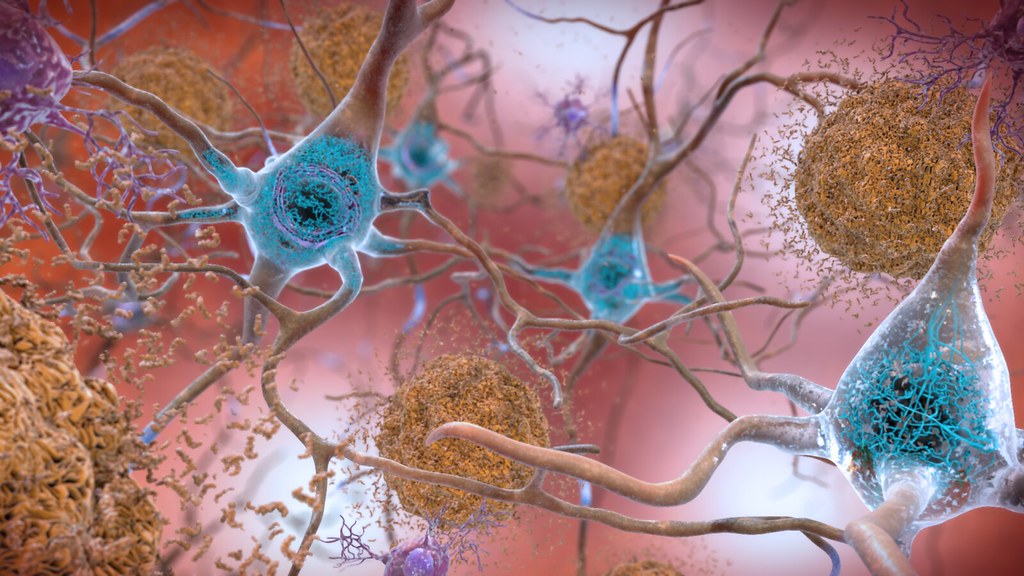
Introduction to proteins: the workhorses of life
Within the crowded interior of each cell, proteins are the workhorses of life. These complex molecules carry out a staggering number of tasks, from building tissues and fending off disease-causing agents to moving essential substances and driving intricate thought processes. For a protein to perform its particular function, it needs to carefully fold into a specific three-dimensional (3D) shape, a specific conformation that will define its function and is crucial to its proper functioning1. But if these native conformation of proteins gets disrupted, it marks the beginning of protein misfolding and aggregation, processes that usually lead to significant health issues, most notably through the creation of specific, problematic aggregates called amyloids2.
The delicate dance of protein folding
It’s not always easy for a protein to achieve that ideal 3D shape. Proteins are not just long strings of amino acids; they have to fold themselves into a precise structure, undergoing a great deal of bending, twisting, and compacting in the process. Some proteins can fold autonomously, discovering their optimum conformation without any help, but many others require a crucial helping hand3.
That’s where molecular chaperones enter the picture4. These vital cellular “helpers” function like watchful guides, monitoring proteins to fold properly and keeping them from taking “shortcuts” that could cause problems. They’re crucial for keeping our cellular protein workforce healthy, working hard to keep them from misfolding and even refolding proteins that have strayed. There are many such well-studied families, such as the Hsp70 and Hsp90 proteins5.
When and why protein misfolds
Despite the cell’s best efforts, there are times when things go wrong. Protein misfolding happens when a protein fails to achieve or sustain its correct structure6. This can occur for a variety of reasons.
- At times, it is a result of genetic mutations, when an alteration in the DNA blueprint changes the sequence of amino acids and makes it more difficult for the protein to fold7.
- Environmental stresses such as high temperature, changes in pH, or even oxidative stress may also interfere with the stability of a protein8.
- As we get older, our cells’ machinery becomes less effective, and misfolding becomes more likely.
- Even simply having too many copies of an individual protein will overburden the folding mechanism9.
When proteins misfold, they can either lose their desired function, or worse, acquire new, toxic properties10.
From misfolding to aggregation
A misfolded protein tends to have “sticky” areas, usually hidden within its structure, that become exposed11. The sticky spots are like molecular Velcro, and they make misfolded proteins clump or aggregate together. This clumping is not merely a random pile; it can adopt different aggregate forms, from cross-linked amorphous dumps to highly ordered ones. What we’re most interested in is a specific, often damaging, progression.
It begins with single protein units (monomers) coming together in tiny, soluble clumps known as oligomers12. These are especially toxic, frequently regarded as the most dangerous forms, behaving like molecular seeds. They may then develop into larger, more organized structures called protofibrils, and ultimately, into mature, insoluble fibrils12. This development is a critical step towards the creation of amyloid structures, which we will explore next.
Amyloids: ordered aggregates with pathological potential
Of all the forms of protein aggregates, amyloids stand out because of their very organized and stubborn nature. They have a distinctive “cross-β” sheet fold, a typical protein structure that has a ribbon-like, pleated appearance, making them extremely stable, insoluble, and highly resistant to the cell’s normal housekeeping processes13. These structures are similar to dense, tightly packed, impenetrable fibers. Their presence can be identified by their ability to bind certain dyes, such as Thioflavin T and Congo Red, a characteristic feature employed in diagnosis and research14, 15.
Quite a few severe neurodegenerative disorders are pathologically defined by the formation of specific amyloid-forming proteins. Examples include alpha-synuclein (α-syn) in Parkinson’s Disease and other synucleinopathies, amyloid-beta (Aβ) plaques and tau protein tangles in Alzheimer’s Disease, Prion protein (PrP) in fatal prion diseases, and huntingtin protein in Huntington’s Disease16-19. All form unique amyloid aggregates associated with their respective disorders.
Cellular defenses and when they fail
Our cells are not defenseless against misfolded proteins. They possess a complex protein quality control (PQC) system that works to preserve protein health20. Molecular chaperones, in addition to aiding folding, are also capable of attempting refolding of misfolded proteins or even assisting in breaking apart existing aggregates. When refolding is not an option, the cell resorts to its demolition teams: the ubiquitin-proteasome system, which tags and shreds individual misfolded proteins, and the autophagy-lysosome pathway, which engulfs and recycles bigger protein clumps and broken cellular parts21, 22. But even this robust system can be overwhelmed by too much misfolding, genetic defects in PQC components, or the plain wear and tear that aging causes, resulting in the toxic accumulation of protein aggregates.
The devastating link to neurodegenerative diseases
As already told, the accumulation of certain amyloidogenic proteins is a tragic common thread that lies across numerous neurodegenerative disorders. These aggregates actively alter neuronal structure via a number of disastrous mechanisms23.
They can impair critical cellular activity such as energy production (mitochondrial dysfunction) and communication among brain cells (synaptic dysfunction)24, 25. Additionally, these aggregates can generate destructive oxidative stress and provoke injurious inflammatory processes in the brain. In addition, these misfolded proteins can act in a “prion-like” manner, in that they can convert healthy proteins into their misfolded, aggregated state, essentially “spreading” the disease pathology from one neuron to the next and fueling the advancement of the disease26.
The emerging role of the gut microbiome and biofilms
The tale of protein misfolding is not limited within our cells alone; it encompasses the microscopic world inside us. Bacteria, especially those residing in our gut, synthesize their own amyloid proteins, such as the biofilm-associated proteins (BAPs)27. These BAPs serve a structural function in the dense bacterial aggregates known as biofilms that coat our gut. Importantly, these bacterial amyloids share a similar structure with human amyloid proteins. Such similarity enables cross-seeding, in which bacterial amyloids can serve as templates, facilitating our own host proteins, such as alpha-synuclein, to misfold and aggregate in the gut. From there on, the effects or even the aggregates are thought to pass via the gut-brain axis, potentially along the vagus nerve, leading to neurodegeneration in the brain. An imbalanced gut microbiome, or dysbiosis, can also exacerbate this complex interplay.
Current and future therapeutic strategies
Understanding the science behind misfolding, aggregation, and amyloids is opening the door to thrilling new therapeutic approaches. Scientists are looking into how to strengthen our cells’ own defenses, perhaps by improving chaperone function or enhancing protein destruction pathways. Other strategies aim to prevent aggregation from occurring in the first place, employing small molecules or peptides to block the process28. For aggregates that do form, researchers are working on ways to remove them, for example, with antibodies or specialized enzymes that can degrade them29, 30.
Critically, as increased understanding of the gut-brain axis has come to light, new approaches are being developed that target the gut microbiome itself. These seek to modulate gut dysbiosis (gut bacterial imbalance), inhibit production of microbial amyloids, or block their cross-seeding activity in the gut.
Conclusion
Proteins are the architects of our lives, and proper folding is crucial to our health. When this intricate process goes haywire, leading to misfolding and the formation of toxic amyloid aggregates, the consequences can be devastating, particularly in neurodegenerative diseases. However, our developing understanding of these mechanisms, including the surprising and significant role of the gut microbiome and its own amyloid proteins, offers new hope. By demystifying the complicated science of misfolding, we are unlocking the doors to new diagnostic tools and therapies that potentially will revolutionize the way we prevent and treat these intractable diseases.